1.Research Summary
The Division of Clinical Nephrology and Rheumatology actively conducts
basic and clinical research, as follows:
We are further divided into the following groups: Genome and Molecular
Biology Group; Renal pathology Group; Research Group for Diabetic Nephropathy
and Renal Metabolism; Practical, experimental chronic kidney disease/ uremic
toxin research (PRECURE) Group; and Rheumatology Group. Each group conducts
technically high-level research using a variety of methods including molecular
biology, genetics, biochemistry, histopathology, and epidemiology and also
performs cross-sectional research by sharing research subjects with other
groups.
A research goal of the Genome and Molecular Biology Group is to elucidate
the underlying molecular mechanism of the pathogenesis and to prevent the
progress of kidney diseases. This group strives to investigate them from
the aspects of molecular, cellular to systemic-level dysfunction by introducing
the study techniques of genomics and proteomics and the animal models mimicking
kidney diseases.
The Renal pathology Group is in charge of the diagnosis of renal biopsy
specimen collected from all our associated facilities in Niigata Prefecture
and provides histological information to clinician. This group conducts
research on renal transplant pathology in collaboration with the Department
of Urology. In addition, this group studies new conditions based on pathological
diagnosis.
The Research Group for Diabetic Nephropathy and Renal Metabolism approaches
the pathology and complications of diabetes mellitus in terms of renal
metabolism and studies the role of proximal tubule cells in particular.
The group analyzes endocytic receptor molecules and studies the etiologies
of diabetic nephropathy and nephropathy associated with metabolic syndrome
based on these analyses.
The Practical, experimental chronic kidney disease/ uremic toxin research
(PRECURE) Group studies CKD-induced various complications, such as bone
fractures and cardiovascular disease. Those events increase with CKD progress;
however, the mechanisms are incompletely understood. We try to elucidate
them with both basic and clinical research, and make strategies to improve
their QOL and ADL.
The Rheumatology Group is currently conducting research on the following:
the pathology and therapy of nephropathy due to reactive amyloidosis in
patients with rheumatoid arthritis; vascular lesions and arteriosclerotic
lesions in patients with connective tissue diseases; and microfractures
in patients with connective tissue diseases who are on oral bisphosphonate
therapy. This group also performs psychosomatic research on the factors
precipitating and ameliorating pain and physical symptoms in rheumatic
and connective tissue disease patients.
2. Research Groups
3. The Nephrology and Rheumatology has the following subgroups as research teams
1) Genome and Molecular biology Group
Research subjects
Genome analysis of the development and progression of kidney diseases (focusing
particularly on IgA nephropathy)
Effect of aging on the development and progression of kidney disease using
mouse model
2) Renal pathology Group
Research subjects
Pathological diagnosis by renal biopsy—clinicopathological study
about the onset and during progression of the primary glomerulonephritis
and secondary nephropathy
Clinicopathological study of renal transplant rejection, and recurrent
or de novo nephritis after renal transplantation
Long-term follow-up of IgA nephropathy
3) Research Group for Diabetic Nephropathy and Renal Metabolism
(Collaborated with Department of Applied Molecular Medicine and Department of Clinical Nutrition Science)
Research subjects
Clinical and basic research on diabetic nephropathy
Functional analysis and clinical application of megalin in the proximal
tubule
Research on diet therapy in CKD
Research on protein and lipid metabolism in CKD
Research on drug-induced nephrotoxicity
Analysis of renin-angiotensin system in the kidney
4) Practical, experimental chronic kidney disease/ uremic toxin research (PRECURE)
Research subjects
Clinical studies of risk factors for CKD progression
Clinical studies of mortality and CKD-related complications in CKD patients,
especially undergoing dialysis treatment
Mechanisms for CKD-induced acceleration of atherosclerosis, especially
focused on uremic toxins
Mechanisms for CKD-mineral bone disorders and uremic osteoporosis
Pathogenesis of dialysis-related amyloidosis
Development of new blood purification system for more removal of uremic
toxins
Convenient blood purification system at disaster for hemodialysis patients
Study about vascular access hemodialysis catheters
5) Rheumatology Group
Research subjects
Reactive amyloidosis associated with rheumatoid arthritis
Idiopathic osteonecrosis of femoral head in patients taking glucocorticoid
Atypical femoral fractures in patients with connective tissue diseases
taking oral bisphosphonates
Long-term prognosis of patients with SLE / ANCA-associated vasculitis
Clinical characteristics of ANCA-associated otitis media
4.Research Results
[Area] Genome and Molecular Biology Group
[Research subject]
Bacterial composition in tonsillar crypts in patients with IgA nephropathy.
[Description]
IgA nephropathy is a primary chronic glomerular disease that is characterized
pathologically by deposits of galactose-deficient IgA1 and the mesangial
proliferation. However, how galactose-deficient IgA1 is produced and deposits
in mesangium remains to be elucidated.
IgA nephropathy patients sometimes present temporary exacerbation of hematuria
at the time of tonsillar infection, and the efficacy of tonsillectomy for
the treatment of IgA nephropathy has been reported. Therefore, it has been
speculated that the bacterial flora present in tonsils causes some immunological
modification in mucosal immunity and leads to the development of IgA nephropathy.
The cultivation and isolation of bacteria have long been the gold standard
for the identification and characterization of microbes. However, 99% or
more of the bacteria are thought to be difficult to cultivate, which was
an obstacle to detailed research of bacterial flora. We performed the comprehensive
microbiome analysis in the palatine tonsils of IgA nephropathy patients,
collaborating with Department of Otolaryngology Head and Neck Surgery in
Niigata University, in a joint research with Tokyo Institute of Technology,
National Institute of Genetics, and Juntendo University. Genomic DNA from
tonsils of each patient was extracted, and the 16S ribosomal RNA gene was
amplified and analyzed using a high-throughput multiplexed sequencing approach.
We found that the patterns of bacteria in tonsils of IgA nephropathy patients
are similar with those of recurrent tonsillitis patients. The host response
to the bacteria might be important in the development of IgA nephropathy.
The results were reported in Nephrology Dialysis Transplantation.
[Photographs]
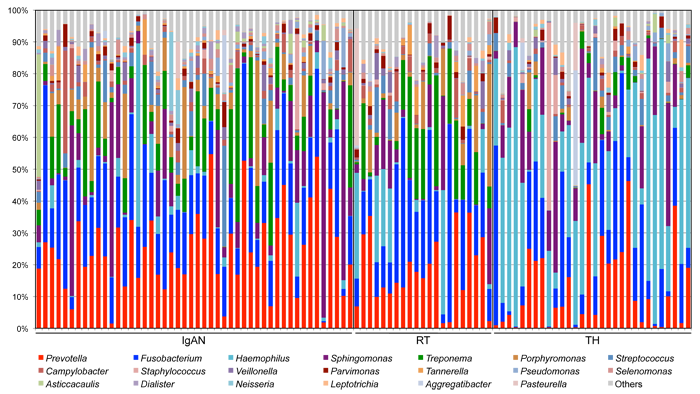
Bacterial composition in tonsillar crypts by 16S rRNA gene sequencing. IgAN, IgA nephropathy patients; RT, Recurrent tonsillitis patients; TH, Children with tonsillar hyperplasia.
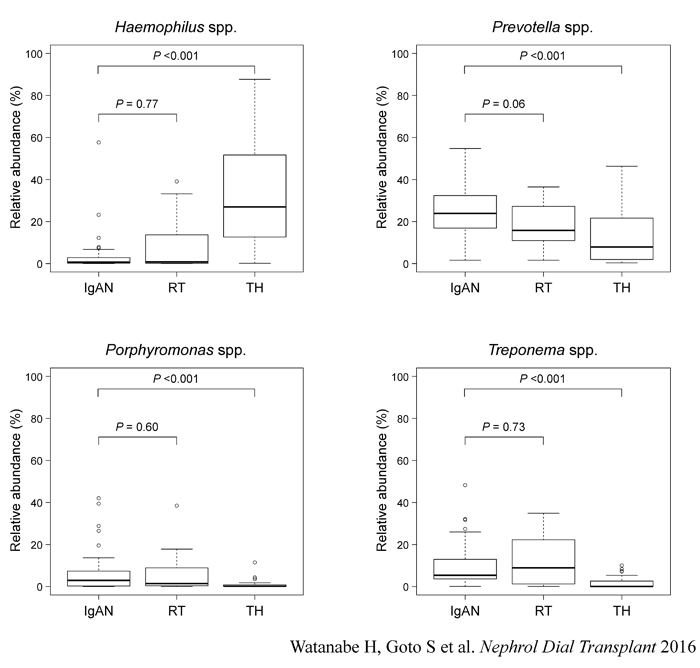
Comparison of the relative abundance of each genus.
[Area] Renal pathology Group
[Research subject]
Light-microscopic characteristics of IgG4-related kidney disease
[Description]
IgG4-related kidney disease (IgG4-RKD) is defined as renal lesions of IgG4-related
disease, which is a new disease concept originating in Japan that is characterized
by increased serum IgG4 levels and tissue infiltration of IgG4-positive
plasma cells, and often presents with tubulointerstitial nephritis. We
conducted a multicenter collaborative study with Kanazawa University, Kobe
University, Joetsu University of Education, and Nagaoka Red Cross Hospital
to reveal light-microscopic differences between IgG4-RKD and other forms
of tubulointerstitial nephritis. Specific interstitial fibril formation
(storiform fibrosis, figure on the right) and cellular infiltration expanding
into the renal capsule were observed only for IgG4-RKD. We concluded that
these findings are crucial to distinct IgG4-RKD from other forms of interstitial
nephritis.
Look at reference materials
[Photographs]
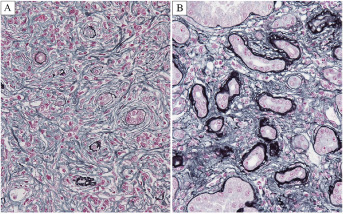
Fig. 3. Interstitial fibrosis of IgG4-related TIN and non-IgG4-related TIN. Characteristic storiform fibrosis is evident in IgG4-related TIN (A) but not in non-IgG4-related TIN (B) (PAM Masson trichrome, X400).
[Area] CKD Pathology Group
[Research subject]
Uremic toxins-induced atherosclerosis and the therapeutic intervention
[Description]
Advanced kidney disease increase cardiovascular event with progressive
atherosclerosis as well as vascular calcification. Macrophage foam cell
formation induced by functional abnormalities of macrophage and HDL cholesterol
is one of pivotal roles for atherogenesis, and we focus on the effect of
uremic toxins accumulated with kidney disease.
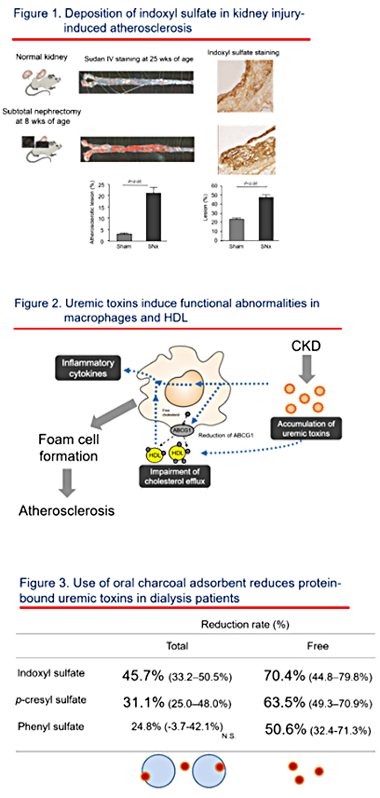
Indoxyl sulfate, one of uremic toxins with high protein-bound property,
is accumulated in atherosclerotic lesion accelerated by kidney damage in
a mouse model (Yamamoto S, Nephrol Dial Transplant 2011, Figure 1). In
vitro study, indoxyl sulfate increases macrophage inflammatory cytokine
production as well as reactive oxygen species (Matsuo K, Toxins 2015).
Indoxyl sulfate also impair macrophage cholesterol efflux to normal HDL
with less expression of ABC transporter (Matsuo K, Toxins 2015). HDL from
kidney disease patients impairs lipid acceptor function from normal macrophages
(Yamamoto S, J Am Coll Cardiol 2012). These results suggest that kidney
disease, especially accumulation of uremic toxins including indoxyl sulfate,
induces functional abnormalities both of macrophages and HDL which will
lead to macrophage foam cell formation (Yamamoto S, Clin Chem Acta 2016,
Figure 2).
Clinically, it is difficult to remove enough amount of uremic toxins with
high protein-bound property using conventional hemodialysis treatment and
we believe increase of uremic toxin removal will improve quality of life
and survival in dialysis patients. We showed effect of using oral charcoal
adsorbent (Yamamoto S, Sci Rep 2015, Figure 3) and a direct hemoperfusion
with hexadecyl-immobilized cellulose beads (Yamamoto S, Artificial Organs
in press) on the removal of protein-bound uremic toxins, and now are trying
to develop a novel blood purification system.
[Photographs]
[Area] Rheumatology Group
[Research subject]
Significant association between renal function and area of amyloid deposition in kidney biopsy specimens in both AA amyloidosis associated with rheumatoid arthritis and AL amyloidosis.
[Description]
The purpose of this study was to clarify the difference in clinical features
between AA and AL amyloidosis by the difference in the amount and distribution
of amyloid deposition in the renal tissues. 58 patients with an established
diagnosis of AA amyloidosis (AA group) and 61 with AL amyloidosis (AL group)
were retrospectively investigated the correlation between clinical data,
pathological manifestations, and the area occupied by amyloid in renal
biopsy specimens. Serum creatinine, creatinine clearance (Ccr) and estimated
glomerular filtration rate (eGFR) indicated significant renal impairment
in the AA group, whereas urinary protein indicated significant renal impairment
in the AL group. Pathological examinations revealed the different deposit
patterns of amyloid in glomerular basement membrane, the mesangial area,
and glomerular capillary between AA and AL groups, suggesting the cause
of the different patterns of kidney dysfunctions in these 2 groups. (Kuroda
T et al. Amyloid. 2017 Jun;24(2):123-130.)
[Photographs]
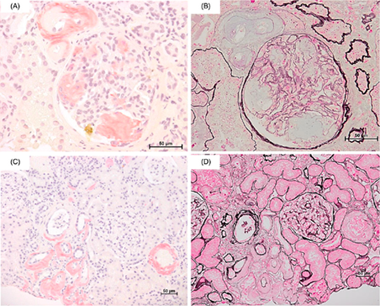
Figure 1. amyloid deposition patterns in AA amyloidosis.
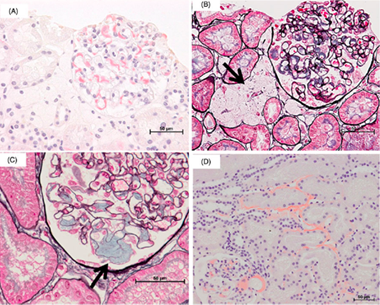
Figure 2. amyloid deposition patterns in AL amyloidosis.
