Measurements and analyses of nanometer-scale motions in the cochlear sensory epithelium (click here to go back to "Research")
【Outline of the research project】
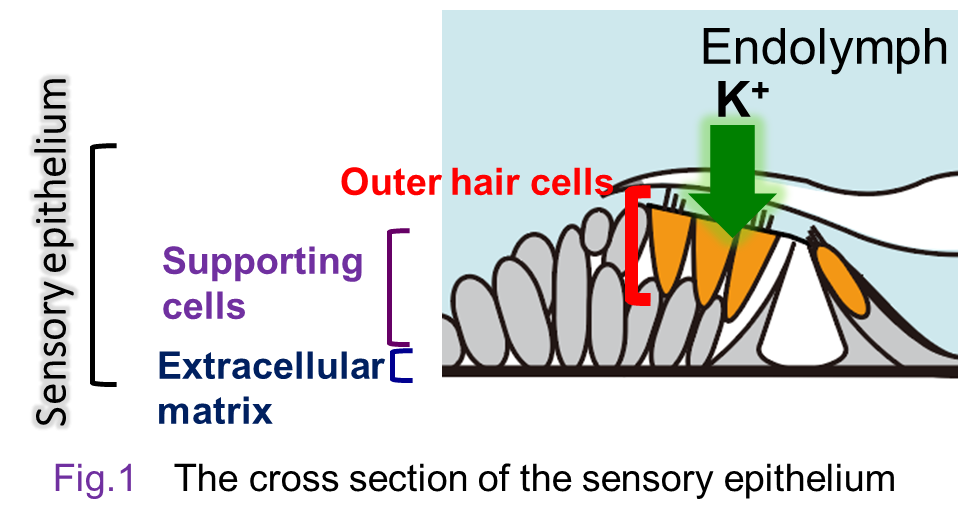 The cochlea of the inner ear has the sensory epithelium, an essential
tissue made up of three layers, i.e., hair-cell and supporting-cell layer
and the basilar membrane (Fig. 1). Acoustic inputs firstly stimulate the basilar membrane and vibrates the sensory epithelium. This event deflects the stereocilia of the hair cells and further activates ‘mechanoelectrical transduction channels’ at their tips. The channel’s opening enters K+ in the endolymph into the hair cells and electrically excites them. In this process, mechanical energy of sounds is transduced to electrical signals. For the details of the process, see the section ‘Mechanism underlying
transmission of acoustic signals in the inner ear’(→link).
The cochlea of the inner ear has the sensory epithelium, an essential
tissue made up of three layers, i.e., hair-cell and supporting-cell layer
and the basilar membrane (Fig. 1). Acoustic inputs firstly stimulate the basilar membrane and vibrates the sensory epithelium. This event deflects the stereocilia of the hair cells and further activates ‘mechanoelectrical transduction channels’ at their tips. The channel’s opening enters K+ in the endolymph into the hair cells and electrically excites them. In this process, mechanical energy of sounds is transduced to electrical signals. For the details of the process, see the section ‘Mechanism underlying
transmission of acoustic signals in the inner ear’(→link).
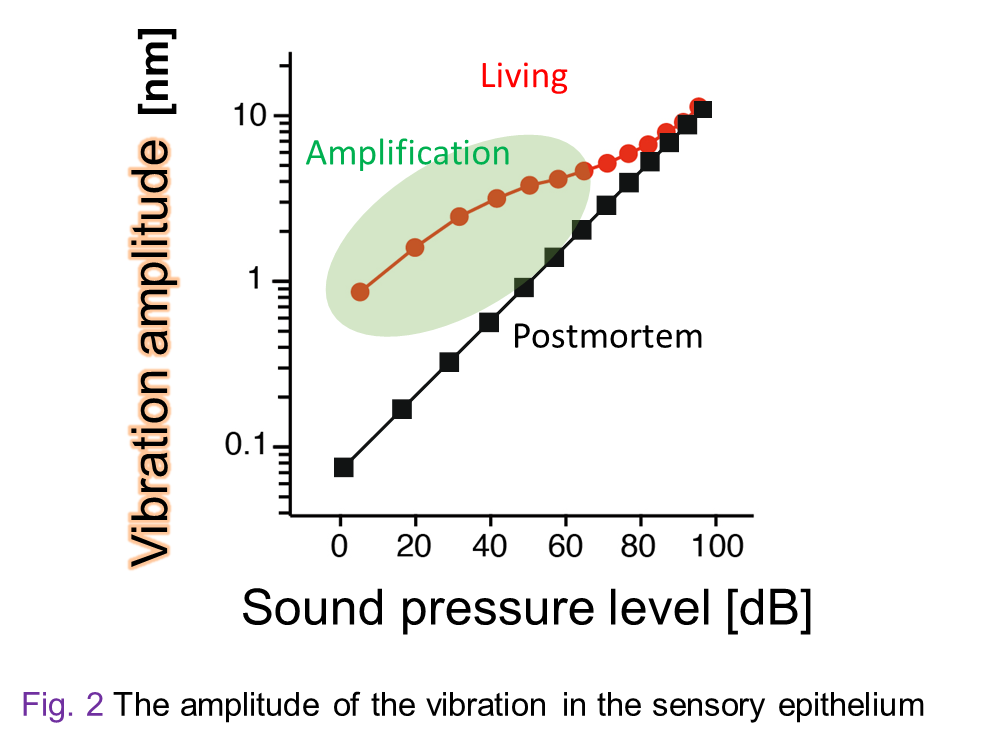
Our hearing can enhance small sounds and perceive them with higher sensitivity, whereas it detects loud sounds with lower sensitivity. This property emerges primarily from a pattern of the motion in the sensory epithelium. Figure 2 plots the amplitude of epithelium’s vibration as a function of intensity of the acoustic stimuli. The relationship described in this panel clearly represents the aforementioned property. This is referred to as ‘nonlinear response’, which disappears when the animal is dead. Note that the amplitude of the vibration in the epithelium is very subtle and ranges from 0.1 nm at minimum to 10 nm at maximum (Fig. 2); the difference is 100-fold. Your hearing can sense a buzzing of a mosquito that flies 3 m away from you as well as accept a jet engine sound emitted form an aircraft nearby. This difference of 1,000,000-fold in sound pressure levels is highly compressed to 100-fold in the motion in the sensory epithelium. The large dynamic range results from the nonlinear response mentioned above and this profile is crucially involved in establishment of the sharp tuning of our hearing.
 Hair cells isolated from the cochlea can change their shape in response to electrical stimuli. This element is likely to crucially contribute to the nonlinear response in the sensory epithelium. Nevertheless, it remains unclear ‘in vivo’ how the hair cells move and their motion are associated with vibrations
in the supporting-cell layer and/or basilar membrane. To address this issue,
we are developing an advanced laser interferometer and a unique optical coherence tomography (OCT) together with engineers and trying to investigate the mechanism underlying the characteristic vibrations in the sensory epithelium.
For details, see the page of ‘Analytical instruments’ and homepage of our collaborator, Dr. Samuel Choi.
Hair cells isolated from the cochlea can change their shape in response to electrical stimuli. This element is likely to crucially contribute to the nonlinear response in the sensory epithelium. Nevertheless, it remains unclear ‘in vivo’ how the hair cells move and their motion are associated with vibrations
in the supporting-cell layer and/or basilar membrane. To address this issue,
we are developing an advanced laser interferometer and a unique optical coherence tomography (OCT) together with engineers and trying to investigate the mechanism underlying the characteristic vibrations in the sensory epithelium.
For details, see the page of ‘Analytical instruments’ and homepage of our collaborator, Dr. Samuel Choi.


