Development of an advanced drug monitoring system and its applications
(click here to go back to "Research")
【Outline of the research project】
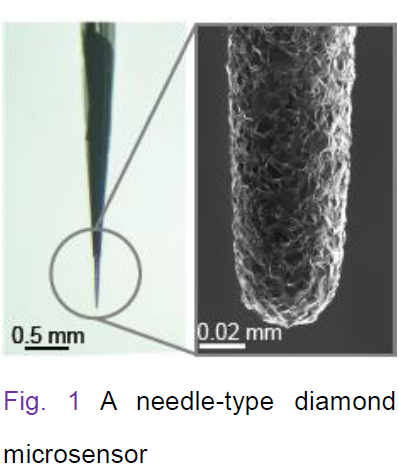 Drugs that are systemically administrated drugs via oral, intravenous, or other routes are diffused to all the organs such as brain and heart. An organ is made up of a number of ‘small’ cell groups, each of which has different properties and roles. Majority of diseases are triggered by impairment of one or some of these cell groups. To clarify the mechanisms underlying beneficial and adverse effects of drugs requires determining whether the compounds actually reach the target cell group and how their concentrations (i.e., pharmacokinetics) as well as reactions on cellular functions (i.e., pharmacodynamics) change over time. Nevertheless, these parameters have not yet been simultaneously measured in in vivo microenvironments by any conventional techniques.
Drugs that are systemically administrated drugs via oral, intravenous, or other routes are diffused to all the organs such as brain and heart. An organ is made up of a number of ‘small’ cell groups, each of which has different properties and roles. Majority of diseases are triggered by impairment of one or some of these cell groups. To clarify the mechanisms underlying beneficial and adverse effects of drugs requires determining whether the compounds actually reach the target cell group and how their concentrations (i.e., pharmacokinetics) as well as reactions on cellular functions (i.e., pharmacodynamics) change over time. Nevertheless, these parameters have not yet been simultaneously measured in in vivo microenvironments by any conventional techniques.
 We have recently developed an advanced drug monitoring system equipped
with a needle-type ‘diamond microsensor’ (Fig. 1) and succeeded in detecting local pharmacokinetics and pharmacodynamics
of a few drugs in live anesthetized animals. This work has been published
in Nature Biomedical Engineering (see our publication list below) and is conducting by our close collaboration
with the group of Professor Einaga who is an engineer in Keio University
and a pioneer for diamond electrodes. Our system and methodology will contribute
to improvement of administration regimens in such that the drugs can react
on the target cells or tissues maximumly with minimizing the adverse effects
as well as accelerate safe drug development.
We have recently developed an advanced drug monitoring system equipped
with a needle-type ‘diamond microsensor’ (Fig. 1) and succeeded in detecting local pharmacokinetics and pharmacodynamics
of a few drugs in live anesthetized animals. This work has been published
in Nature Biomedical Engineering (see our publication list below) and is conducting by our close collaboration
with the group of Professor Einaga who is an engineer in Keio University
and a pioneer for diamond electrodes. Our system and methodology will contribute
to improvement of administration regimens in such that the drugs can react
on the target cells or tissues maximumly with minimizing the adverse effects
as well as accelerate safe drug development.
【Experimental procedures and results】
 Size of cell group is often less than 1 mm. Our drug monitoring system
harbors two different sensors. One is a needle-type ‘diamond microsensor’
(tip diameter: ~40 µm, Fig. 1), which can detect local drug concentrations over time. As compared with conventional materials for electrochemical sensors such as carbon, gold, and platinum, diamond has several advantages including a wide dynamic range detecting a variety of compound types, rapid and stable response, and low noise. The other sensor is a ‘glass microelectrode’ (tip diameter: ~1 µm, Fig. 2), which directly measures cellular electrical activity. Note that this element is the target for 15% of the drugs applied to patients at clinical sites. In our study, we inserted these two microsensors at the vicinity of a particular cell group in different organs and succeeded in monitoring behaviors of drugs and cellular functions ‘simultaneously and in real time’.
Size of cell group is often less than 1 mm. Our drug monitoring system
harbors two different sensors. One is a needle-type ‘diamond microsensor’
(tip diameter: ~40 µm, Fig. 1), which can detect local drug concentrations over time. As compared with conventional materials for electrochemical sensors such as carbon, gold, and platinum, diamond has several advantages including a wide dynamic range detecting a variety of compound types, rapid and stable response, and low noise. The other sensor is a ‘glass microelectrode’ (tip diameter: ~1 µm, Fig. 2), which directly measures cellular electrical activity. Note that this element is the target for 15% of the drugs applied to patients at clinical sites. In our study, we inserted these two microsensors at the vicinity of a particular cell group in different organs and succeeded in monitoring behaviors of drugs and cellular functions ‘simultaneously and in real time’.
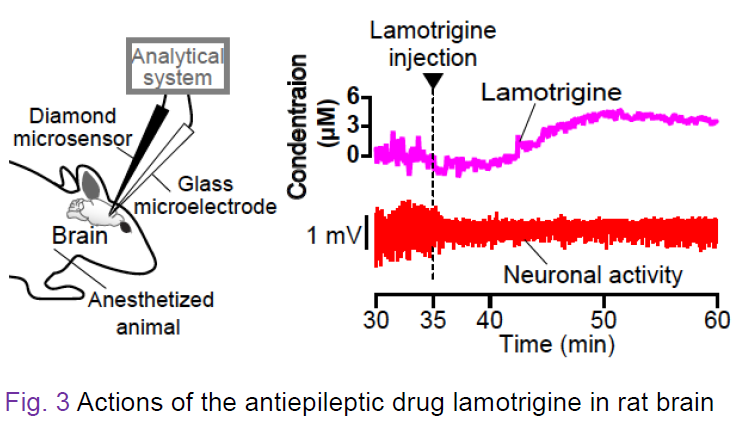
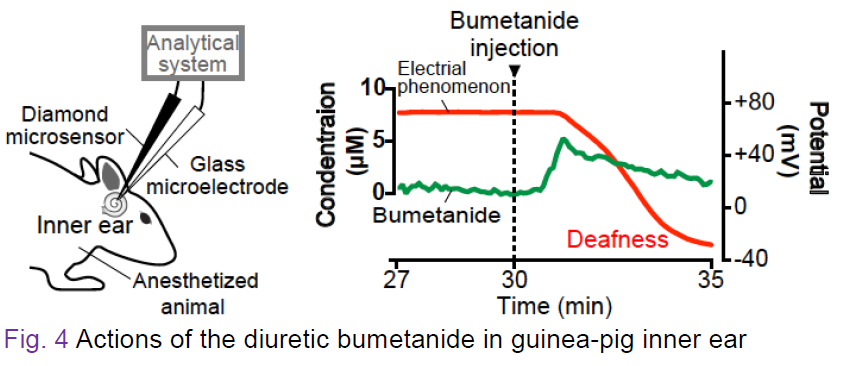 Figure 3 illustrates the actions of the antiepileptic drug lamotrigine that was
intravenously injected in a rat. The two microsensors were placed in the
brain. As shown in the right panel, as soon as the lamotrigine concentration
increased (purple), the neural activity was markedly suppressed (red). Thereafter, the drug behaved slowly, and 15 min after the injection the concentration began to decrease.。
Figure 3 illustrates the actions of the antiepileptic drug lamotrigine that was
intravenously injected in a rat. The two microsensors were placed in the
brain. As shown in the right panel, as soon as the lamotrigine concentration
increased (purple), the neural activity was markedly suppressed (red). Thereafter, the drug behaved slowly, and 15 min after the injection the concentration began to decrease.。
Figure 4 indicates the actions of bumetanide that is applicable to hypertension and epilepsy treatments but sometimes induces deafness by impairing electrical phenomenon in the inner ear. In the experiment, this diuretic was intravenously injected in a guinea pig and its pharmacokinetics and pharmacodynamics were monitored with the sensors inserted into the snail-shaped inner ear. The right panel depicts the results. Immediately after the injection the drug concentration was rapidly elevated and 1 min later it began to be reduced (green). Simultaneously recorded electrical phenomenon (i.e., potential; red) started to be impaired around when bumetanide concentration reached the peak. The kinetics of this drug is clearly different from that of lamotrigine shown in Figure 3.
In addition, we monitored the actions of the anticancer reagent doxorubicin in vivo. Moreover, we found that our drug monitoring system can offer the potential to detect some antidepressants and antibiotics and other anticancer compounds in different organs. Therefore, this state-of-the-technology has a generality in the applications.
Perspectively, this system would contribute to issues as follows.
(1) Development of safe and effective drugs.
(2) Proposal of administration regimens that permit the drugs to induce beneficial effects with minimal adverse actions.
(3) Advances in drug depositioning.
(4) Acceleration of personalized medicine.
Recent studies using advanced technologies such as DNA microchips have revealed SNPs may at least partially account for difference in the drug actions among individuals. Furthermore, a number of researchers are working on development of drug-delivery systems that allow the chemical compounds to reach the target cells and tissues. However, in different occasions and situations, it remains uncertain how the drugs behave and their actions change in target cells or microenvironments in vivo. This issue, which is essential for medicine in next generation, could be addressed by our technology and methodology.
【Publication list】
【Feature articles】
(1) Alan D (2018). In vitro veritas: Biosensors and microarrays come to life. Science 359:1287-1290.


