Digestive and General Surgery
HOME > Activities > Clinical Medicine > Digestive and General Surgery
1.Research Summary
At Niigata University Graduate School of Medicine and Dental Sciences,
we are divided into the following two departments, both of which conduct
highly technical research. With the two departments working together cooperatively,
we also implement research in which surgical oncology and regenerative
medicine are combined.
- 1)Course for Molecular and Cellular Medicine/Gene Control/Surgical Oncology
Department
- 2)Course for Biological Functions and Medical Control/Regenerative Medicine/Gastrointestinal
and General Surgery Department
2.Research Groups by Organ
- Hepato-Biliary-Pancreatic Group
- Esophagus and Gastric Surgery Group
- Colorectal Surgery Group
- Breast and Endocrine Surgery, and Surgical Metabolism and Nutrition Group
3-1.Research subjects of Hepato-Biliary-Pancreatic Group
- Clinicopathological study of the mode of spread from biliary tract cancer
(see research results)
- Clinical significance of surgery for recurrent biliary and pancreatic cancer:
a multi-institutional study
- Clinical significance of total pancreatectomy: a multi-institutional study
- DNA damage response in malignant hepato-biliary-pancreatic tumor
- Practice and immunological study of pancreas and liver transplantation
3-2.Research subjects of Esophagus and Gastric Surgery Group
- Clinical study on adjuvant chemotherapy for curatively resected Stage III
gastric cancer
- Clinical study on multidisciplinary treatment performed with chemoradiotherapy
in esophageal cancer (2 studies)
- Effect of gastrectomy for gastric cancer with positive cytologic washings
on prognosis
- Effect of esophagectomy for thoracic esophageal cancer on postoperative
respiratory function and QOL
- Clinical studies (5 studies) and clinical trials (4 trials) on genetic
polymorphisms of gastrointestinal stromal tumor (GIST) and molecular targeted
drugs
3-3.Research subjects of Colorectal Surgery Group
- Genomic analysis and its clinical significance in colorectal cancer (see
research results)
- Mechanisms of resistance to anticancer agents in colorectal cancer
- Histopathological findings of the advancing edge of the tumor in colorectal
cancer
- Molecular biology of ulcerative colitis-associated cancer
3-4.Research subjects of Breast and Endocrine Surgery, and Surgical Metabolism
and Nutrition Group
- Comprehensive gene mutation analysis for personalized medicine in breast
cancer
- Study on the interaction between cancer cells and stromal cells in the
tumor microenvironment
- Mechanisms of resistance to anticancer agents in breast cancer
- Analysis of functions of lipid mediators in cancer-specific metabolic control
(see research results)
4.Research Results
|
[Area] Malignant tumor in general (surgical oncology)
|
|
[Research subject]
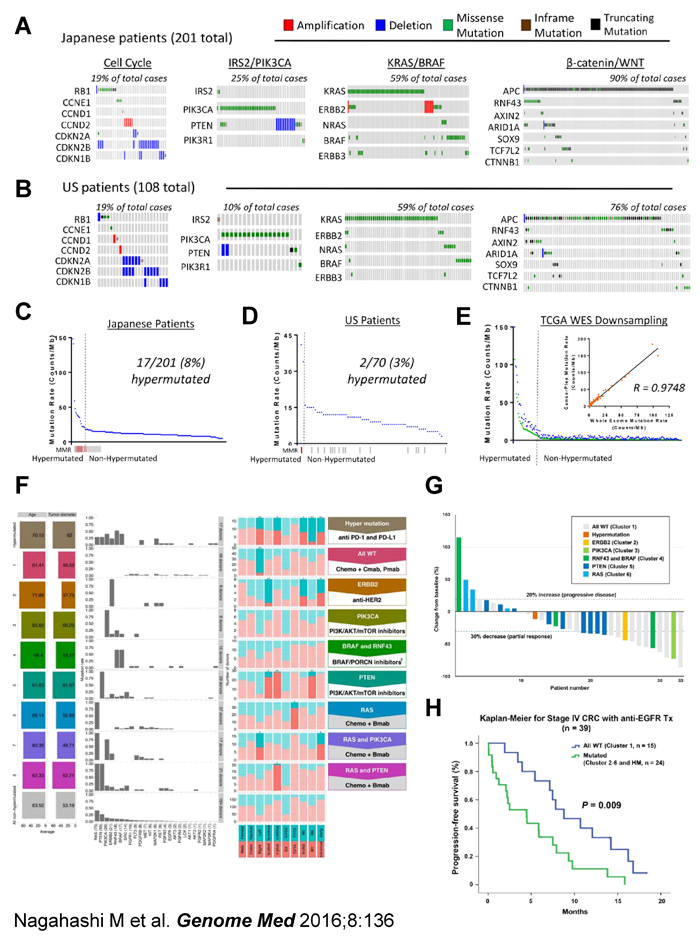
Comprehensive cancer genome profiling of 201 colorectal cancer patients
for realizing precision medicine in Japan
|
|
[Description]
The ultimate goal of cancer genome profiling utilizing next generation
sequencing technology is to enable precision medicine, the tailoring of
treatments based on unique genomic changes of each patient’s individual
tumor. We hypothesized that sequencing a panel of cancer-associated genes
would identify essentially all actionable genomic driver mutations and
further determine mutational burden in colorectal cancer (CRC). In the
current study, we tested this hypothesis utilizing a 415-gene panel designed
for solid tumors at a high depth of coverage (500X) in Japanese patients
(n = 201 tumors) and evaluated for concordance among independent data obtained
from US patients with CRC (n = 108 tumors) (J-CRC and US-CRC respectively)
and from the TCGA-CRC WXS database (n = 224 tumors). We detected clinically
actionable driver mutations in >99% of J-CRC and US-CRC (A). Although
the overall mutation spectrum of the Japanese patients is similar to that
of the Western population, we found significant differences in the frequencies
of mutations in ERBB2 and BRAF (B, C). We further identified tumors with
a hypermutated phenotype in both populations (D-F). Unsupervised clustering
revealed that a panel of 26 genes for targeted therapies can be used to
classify the patients into 8 different categories, each of which can optimally
be treated with a particular combination therapy (F). Waterfall plot analysis
revealed that all the three patients with progressive disease after anti-EGFR
therapies belong to subgroups with actionable driver mutations (G). Moreover,
Kaplan-Meier analysis demonstrated that patients in subgroup of “all wild-type”
showed significantly better progression-free survival as compared to patients
in subgroups of “mutated” (P = 0.009) (H). Use of a panel of 415 genes
can reliably identify all of the critical mutations in CRC patients and
this information can be used to determine the most optimal treatment for
CRC patients.
|
|
[Photographs]
|
|
[Area] Malignant tumor in general (surgical oncology)
|
|
[Research subject]
The role of sphingosine-1-phosphate in the tumor microenvironment and its
clinical implications
|
|
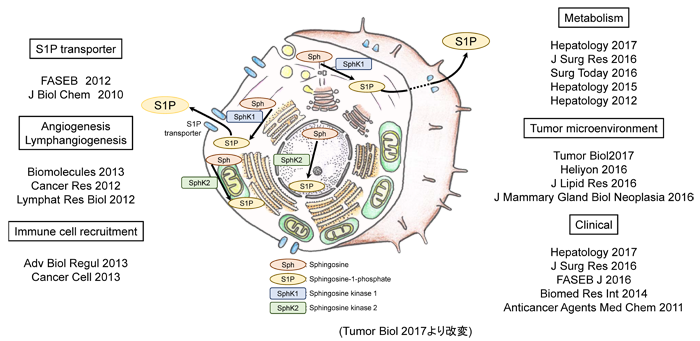
[Description]
Sphingosine-1-phosphate (S1P) is a lipid mediator that functions as a signaling
transductor inside and outside cells like a signaling protein. S1P has
various functions such as proliferation and migration of cancer cells,
angiogenesis and lymphangiogenesis in tumor microenvironment, and induction
of inflammatory cells. Due to these important functions, S1P attracts attention
as a new therapeutic target in cancer. S1P is produced by two isotypes
of Sphingosine kinases (SphK1 and SphK2) in cancer cells and stromal cells
such as the vascular and lymphatic endothelial cells in the tumor microenvironment,
and then is secreted extracellularly and exerts its function.
We have elucidated that SphK1 has important functions in S1P secretion
in breast cancer cells (J Biol Chem 2010) and lymphatic metastasis of breast
cancer (Cancer Res 2012). Since S1P is a lipid, it has been difficult to
measure S1P directly. However, we have successfully measured S1P concentration
of a breast cancer tissue with international research collaboration. We
have revealed that the S1P concentration in the cancer tissues were higher
than in normal breast tissues (J Surg Res 2016) and that S1P concentration
in primary breast cancer tissue is associated with the rate of axillary
lymph node metastasis (J Surg Res 2016). For further investigation of the
significance of S1P in the tumor microenvironment, we have developed a
novel method to collect the interstitial fluid from the tumor tissue and
succeeded to measure S1P concentration in the interstitial fluid (J Mammary
Gland Biol Neoplasia 2016). Furthermore, we have discovered the association
between S1P and chronic intestinal inflammation and colitis-associated
cancer (Cancer Cell 2017).
We have been measuring S1P concentration in various gastrointestinal cancer
tissues such as gastric cancer and pancreatic cancer and analyzing the
clinical significance of S1P. Furthermore, we have generated the latest
animal cancer models, and are analyzing the detailed mechanism how S1P
produced in cancer cells or host microenvironment promotes cancer progression.
Through translational research that connects basic medicine and clinical
practice, we would like to challenge the development of an effective and
safe therapeutic agent targeting the S1P signal in the future.
|
|
[Photographs]
|
|
[Area] Malignant tumor in general (surgical oncology)
|
|
[Research subject]
Relevance of dissection of the posterior superior pancreaticoduodenal lymph
nodes in gallbladder carcinoma
|
|
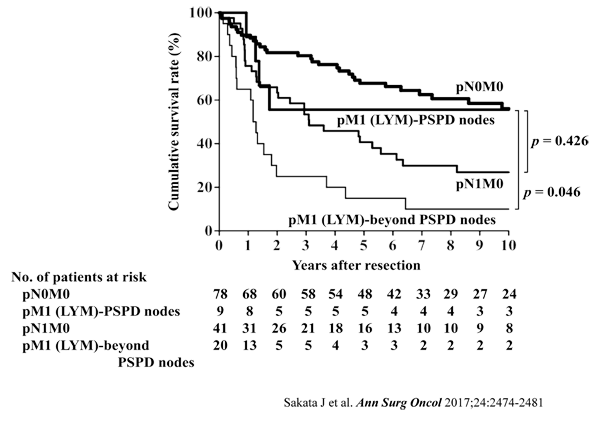
[Description]
The issue of whether the posterior superior pancreaticoduodenal nodes should
be regarded as regional nodes of gallbladder carcinoma remains unresolved.
This study aimed to evaluate the prognostic value of positive posterior
superior pancreaticoduodenal lymph nodes to clarify the need for dissection
of these nodes. A total of 148 patients with gallbladder carcinoma who
underwent radical resection including dissection of the posterior superior
pancreaticoduodenal nodes were enrolled. The incidence of metastasis and
the survival rates among patients with metastasis to each lymph node group
were calculated. Of the 148 patients, 70 (47%) had nodal disease. The incidences
of metastasis in the cystic duct, pericholedochal, retroportal, and hepatic
artery node groups, defined as regional nodes in the UICC TNM staging system,
ranged from 8.3% to 24.3% with 5-year survival rates of 12.5% to 46.4%
in patients with positive nodes. The incidence of metastasis to the posterior
superior pancreaticoduodenal nodes was 12.8% with a 5-year survival rate
of 31.6% in patients with positive nodes. Survival after resection was
significantly better in patients with distant nodal disease affecting only
the posterior superior pancreaticoduodenal nodes (5-year survival, 55.6%)
than in patients with distant nodal disease beyond these nodes (5-year
survival, 15.0%; p = 0.046), whereas survival after resection was comparable
between the former group and patients with regional nodal disease alone
(5-year survival, 40.7%; p = 0.426). In gallbladder carcinoma, involvement
of the posterior superior pancreaticoduodenal nodes is similar to that
of regional nodes in terms of both the incidence of metastasis and the
impact on survival. Inclusion of the posterior superior pancreaticoduodenal
nodes among the regional nodes should be considered.
|
|
[Photographs]
|
Please see the Digestive and General Surgery website for a detailed description of our research.